Pentaerythritol
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,2-Bis(hydroxymethyl)propane-1,3-diol[1] | |
| Other names
2,2-Bis(hydroxymethyl)1,3-propanediol
Pentaerythritol[1] Hercules P 6 Monopentaerythritol Tetramethylolmethane THME PETP Pentaerythrite Pentek Hercules Aqualon improved technical PE-200 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.732 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12O4 | |
| Molar mass | 136.15 g/mol |
| Appearance | white solid |
| Density | 1.396 g/cm3 |
| Melting point | 260.5 °C (500.9 °F; 533.6 K) |
| Boiling point | 276 °C (529 °F; 549 K) at 30 mmHg |
| |
| Solubility |
Slightly soluble in:methanol, ethanol, glycerol, ethylene glycol, formamide; insoluble in: acetone, toluene, heptane, diethyl ether, dichloromethane |
| Vapor pressure | 0.00000008 mmHg (20°C)[4] |
| Hazards | |
| Flash point | 200.1 °C (392.2 °F; 473.2 K) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[4] |
REL (Recommended)
|
TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[4] |
IDLH (Immediate danger)
|
N.D.[4] |
| Related compounds | |
Related compounds
|
Neopentane, Neopentyl alcohol, Neopentyl glycol, Trimethylolethane, Orthocarbonic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
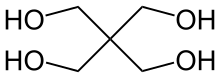
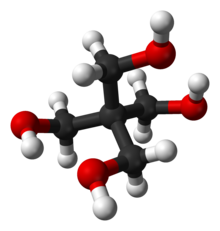
Pentaerythritol is an organic compound with the formula C(CH2OH)4. The molecular structure can be described as a neopentane with one hydrogen atom in each methyl group replaced by a hydroxyl (–OH) group. It is therefore a polyol, specifically a tetrol.
Pentaerythritol is a white solid. It is a building block for the synthesis and production of explosives, plastics, paints, appliances, cosmetics, and many other commercial products.
The word pentaerythritol is a blend of penta- in reference to its five carbon atoms and erythritol, which also possesses 4 alcohol groups.
Synthesis
[edit]Pentaerythritol was first reported in 1891 by German chemist Bernhard Tollens and his student P. Wigand.[5] It may be prepared via a base-catalyzed multiple-addition reaction between acetaldehyde and 3 equivalents of formaldehyde to give pentaerythrose (CAS: 3818-32-4), followed by a Cannizzaro reaction with a fourth equivalent of formaldehyde to give the final product plus formate ion.[6]
Uses
[edit]Pentaerythritol is a versatile building block for the preparation of many compounds,[7] particularly polyfunctionalized derivatives. applications include alkyd resins, varnishes, polyvinyl chloride stabilizers, tall oil esters, antioxidants (e.g. Anox 20). Such derivatives are found in plastics, paints, cosmetics, and many other products. Relevant to resins, pentaerythritol is a precursor to other polyol, such as dipentaerythritol:[8]
- 2 C(CH2OH)4 → O[CH2C(CH2OH)3]2 + H2O
Esters of pentaerythitol are biodegradable,[9][10] and they are used as transformer oils.[11] Due to a very high flash point they also find some use in lubricating gas turbines.[12]
Ester derivatives
[edit]Pentaerythritol is a precursor to esters of the type C(CH2OX)4. Such derivatives are pentaerythritol tetranitrate (PETN), a vasodilator and explosive, the trinitrate derivative pentrinitrol (Petrin), the tetraacetate normosterol (PAG), and the polymer cross-linking agents pentaerythritol tetraacrylate and pentaerythritol tetrakis(3-mercaptopropionate).[13][14]
A linear polymer which can be described as a (spiro) orthocarbonate ester of pentaerythritol, whose formula could be written as [(−CH2)2C(CH2−)2 (−O)2C(O−)2]n, was synthesized in 2002.[15]
Fire retardants
[edit]Pentaerythritol is used as a fire retardant, such as in plastics and intumescent paints and coatings. It releases water upon heating and leaves a deposit of thermally insulating char.[16]
See also
[edit]References
[edit]- ^ a b Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 691. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Yalkowsky, Samuel H. (2010). Handbook of aqueous solubility data (Second ed.). Boca Raton, FL: CRC Press. p. 185. ISBN 9781439802465.
- ^ Yadav, Manish G.; Vadgama, Rajeshkumar N.; Kavadia, Monali R.; Odaneth, Annamma Anil; Lali, Arvind M. (September 2019). "Production of Pentaerythritol Monoricinoleate (PEMR) by immobilized Candida antarctica lipase B". Biotechnology Reports. 23: e00353. doi:10.1016/j.btre.2019.e00353. PMC 6599945. PMID 31304100.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0485". National Institute for Occupational Safety and Health (NIOSH).
- ^ Tollens, B.; Wigand, P. (1891). "Ueber den Penta-Erythrit, einen aus Formaldehyd und Acetaldehyd synthetisch hergestellten vierwerthigen Alkohol (On pentaerythritol, a quaternary alcohol synthetically produced from formaldehyde and acetaldehyde)" (PDF). Justus Liebig's Annalen der Chemie (in German). 265 (3): 316–340. doi:10.1002/jlac.18912650303.
- ^ Schurink, H. B. J. (1925). "Pentaerythritol". Organic Syntheses. 4: 53. doi:10.15227/orgsyn.004.0053; Collected Volumes, vol. 1, p. 425.
- ^ Marrian, S. F. (1 August 1948). "The Chemical Reactions of Pentaerythritol and its Derivatives". Chemical Reviews. 43 (1): 149–202. doi:10.1021/cr60134a004. PMID 18876970.
- ^ Werle, Peter; Morawietz, Marcus; Lundmark, Stefan; Sörensen, Kent; Karvinen, Esko; Lehtonen, Juha (2008). "Alcohols, Polyhydric". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_305.pub2. ISBN 978-3527306732.
- ^ NPCS Board of Consultants & Engineers (2016). The Complete Book on Adhesives, Glues & Resins Technology (with Process & Formulations) 2nd Revised Edition.
- ^ NIIR Board of Engineers & Consultants (2005). Synthetic Resins Technology Handbook.
- ^ Rudnick, Leslie R. (22 December 2005). Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology. ISBN 9781420027181.
- ^ Bhushan, Bharat (28 December 2000). Modern Tribology Handbook, Two Volume Set. ISBN 9780849377877.
- ^ S. F. Marrian (1948). "The Chemical Reactions of Pentaerythritol and its Derivatives". Chemical Reviews. 43 (1): 149–202. doi:10.1021/cr60134a004. PMID 18876970.
- ^ Hoyle, Charles E.; Bowman, Christopher N. (2010). "Thiol–Ene Click Chemistry". Angewandte Chemie International Edition. 49 (9): 1542–1543. doi:10.1002/anie.200903924.
- ^ David T. Vodak, Matthew Braun, Lykourgos Iordanidis, Jacques Plévert, Michael Stevens, Larry Beck, John C. H. Spence, Michael O'Keeffe, Omar M. Yaghi (2002): "One-Step Synthesis and Structure of an Oligo(spiro-orthocarbonate)". Journal of the American Chemical Society, volume 124, issue 18, pages 4942–4943. doi:10.1021/ja017683i
- ^ Stoye, Dieter; et al. (2006). "Paints and Coatings". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a18_359.pub2. ISBN 3527306730.

